Gas Behavior
Gases spread out to fill their containers and change in size and pressure when heated or squeezed.
簡要介紹
Gases are fascinating because they behave very differently from solids and liquids. 🌬️ Think of them as a crowd of energetic bouncing balls that are constantly moving and spreading out. Unlike solids or liquids, gases can be squeezed into smaller spaces or expand to fill larger ones, making them uniquely adaptable to their environment.
主要說明
Movement and Spreading 🏃
Gas particles are always moving randomly and quickly. It's like a group of kids running around in a playground - they spread out to use all the available space and never stay still.
Response to Pressure 👐
When you squeeze a gas (increase pressure), it takes up less space. It's similar to squeezing a spongy stress ball - the more you squeeze, the smaller it gets.
Temperature Effects 🌡️
When gases get hot, their particles move faster and spread out more. Think of popcorn kernels - when heated, they become more energetic and take up more space.
Volume Adjustment 📦
Gases always fill their container completely. It's like pouring water into differently shaped containers - but gases do this even more completely, filling every available space.
範例
- A balloon inflating: As you blow into it, the gas particles spread out to fill the entire balloon, making it bigger 🎈
- Baking bread: The yeast produces gas bubbles that expand when heated, making the dough rise and become fluffy 🍞
- Bicycle tire: When you pump air into a tire, the gas particles get squeezed closer together, creating pressure that makes the tire firm 🚲
費曼AI如何引導你學習
- 選擇任意概念: 從你想掌握的主題開始——瀏覽精選學科或自行輸入。
- 先學核心要點: 用清楚、結構化的解說快速建立知識框架,掌握關鍵與常見誤區。
- 講解並獲得回饋: 以語音或文字錄製你的講解;立即取得在深度、清晰度、結構與示例上的分析。
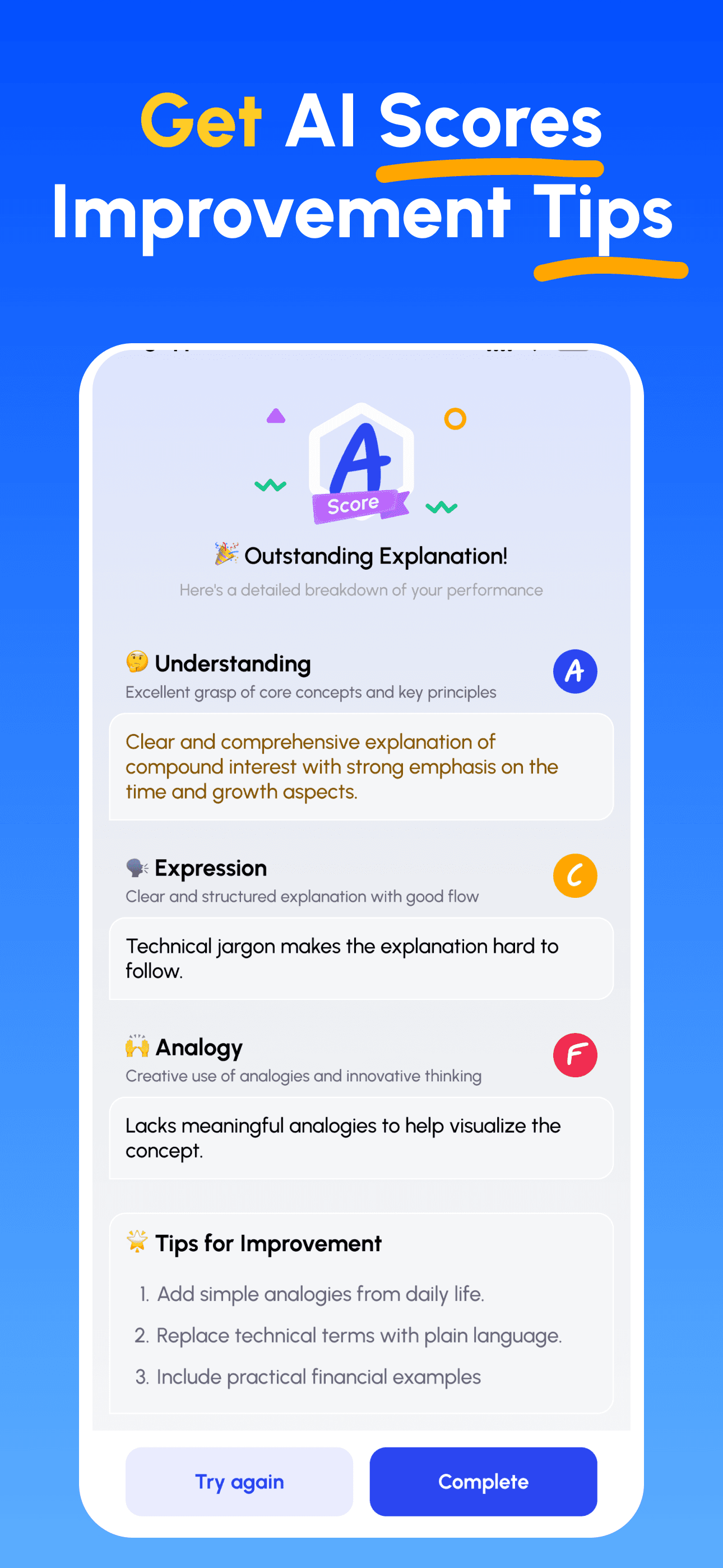
- 檢視評分並精進: 依據針對性建議修正並再講解,直到能簡單講清楚為止。
立即下載費曼AI
今天就開始提升溝通能力的旅程!