Basic Solutions
A basic solution is a liquid mixture that has more OH- ions than H+ ions, making it slippery and bitter-tasting.
簡要介紹
Basic solutions are like the opposite of acidic ones - they're substances that feel slippery, like soap. 🧪 Just like how lemons represent acids, soap and baking soda represent bases. They're important in everyday life, from cleaning products to medicines and food preparation.
主要說明
What makes a solution basic 🔍
A solution becomes basic when there are more hydroxide (OH-) particles than hydrogen (H+) ones floating around in it. It's like having more negative players than positive ones in a game.
Measuring basic solutions 📏
We measure how basic a solution is using the pH scale from 0-14. Anything above 7 is basic - the higher the number, the more basic it is. It's like a thermometer, but for measuring basicness instead of temperature.
Properties of basic solutions 🧼
Basic solutions feel slippery (like soap), taste bitter (like baking soda), and can change certain color indicators (like turning red litmus paper blue). They're often good at cutting through grease, which is why they're used in cleaning.
Common uses 🏠
Basic solutions are everywhere in our homes - in soap, cleaning products, antacids for heartburn, and even in baking. They're helpful because they can neutralize acids and break down certain materials.
範例
- When you wash your hands with soap, you're using a basic solution. The slippery feeling you get is a characteristic of bases. 🧼
- If you've ever taken an antacid for heartburn, you've used a basic solution to neutralize excess stomach acid. 💊
- When you clean an oven with a cleaning spray, you're using a basic solution that helps break down greasy residue. 🧹
費曼AI如何引導你學習
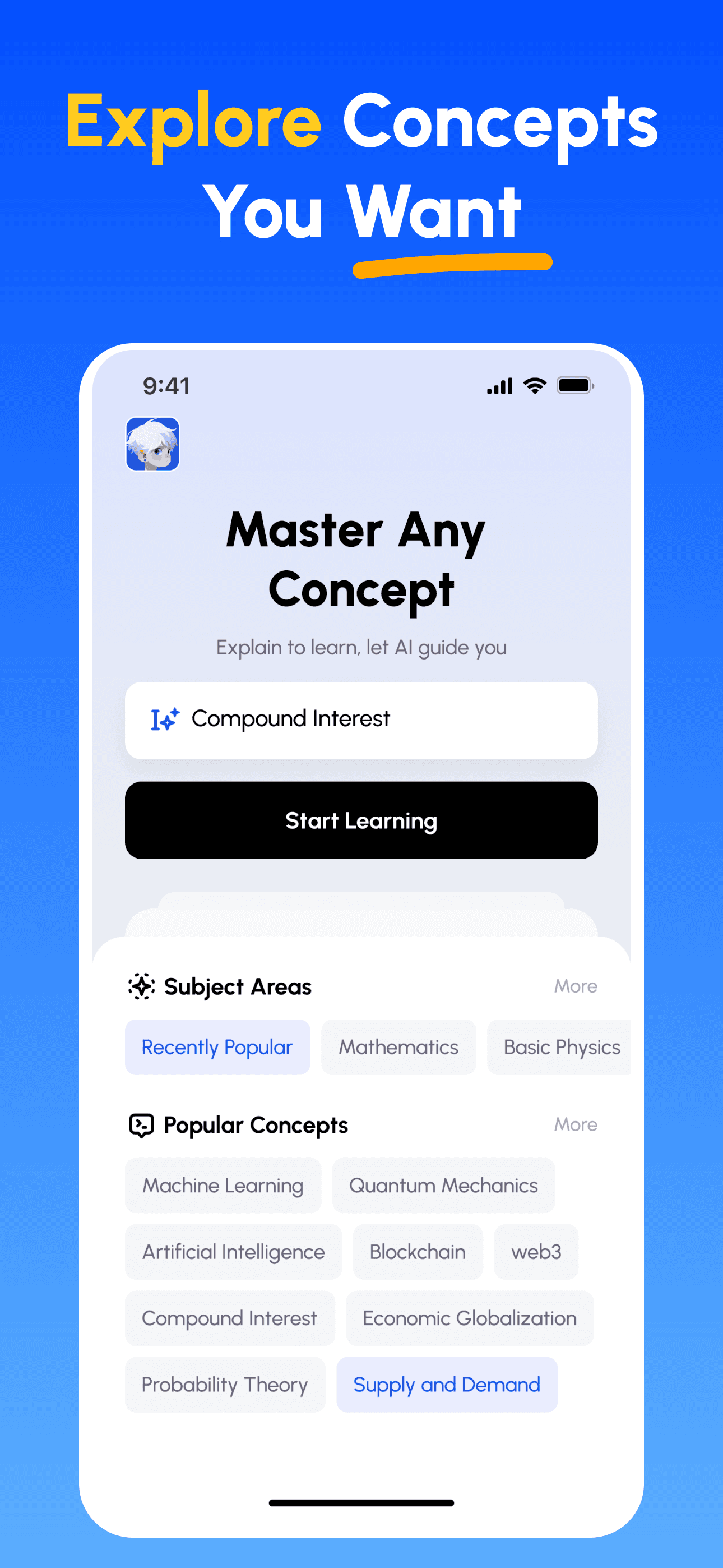
- 選擇任意概念: 從你想掌握的主題開始——瀏覽精選學科或自行輸入。
- 先學核心要點: 用清楚、結構化的解說快速建立知識框架,掌握關鍵與常見誤區。
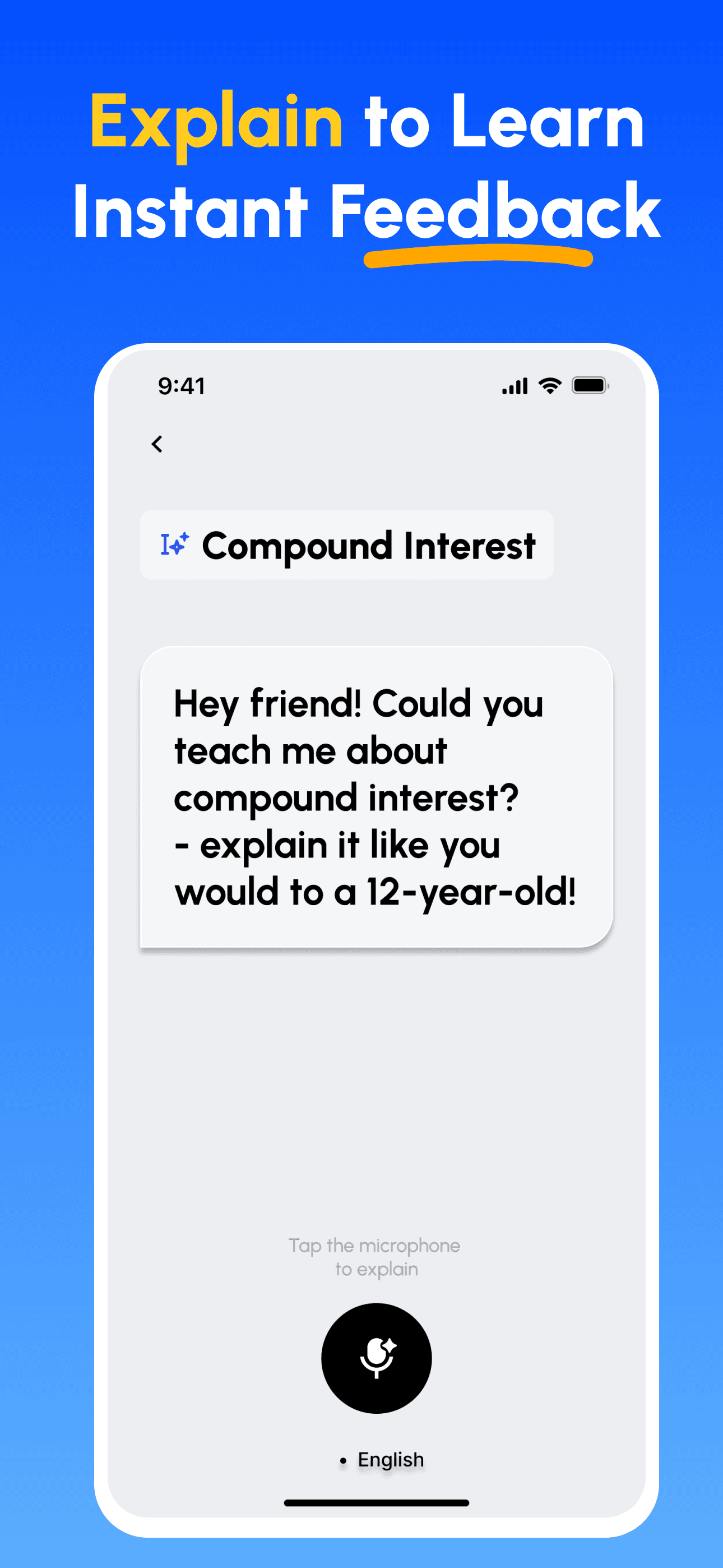
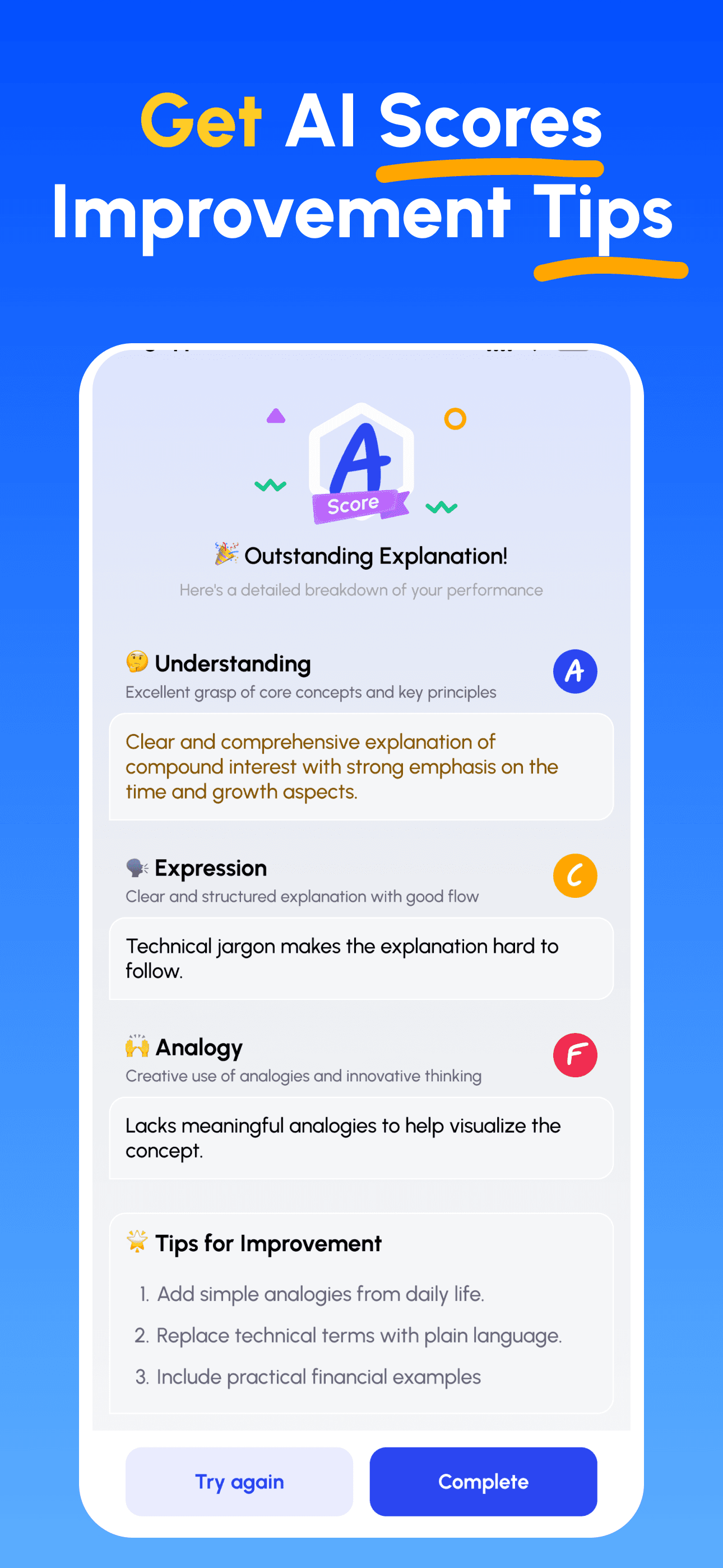
- 講解並獲得回饋: 以語音或文字錄製你的講解;立即取得在深度、清晰度、結構與示例上的分析。
- 檢視評分並精進: 依據針對性建議修正並再講解,直到能簡單講清楚為止。
立即下載費曼AI
今天就開始提升溝通能力的旅程!