Acid and Base
Acids are sour substances that give up protons, while bases are bitter substances that accept protons.
簡要介紹
Acids and bases are two fundamental types of chemicals that we encounter daily, from the citrus fruits we eat to the soap we use. They're like opposite teams in chemistry: acids are substances that can give away hydrogen ions (like sharing a card), while bases are substances that can accept these ions (like collecting cards). Understanding acids and bases helps us make sense of many everyday processes, from cooking to cleaning. 🧪
主要說明
Properties and Behavior
Acids taste sour (like lemons), turn blue litmus paper red, and feel slippery. It's like they're the 'sharp' team. Bases taste bitter (like soap), turn red litmus paper blue, and feel smooth. They're like the 'smooth' team. 🍋
pH Scale
Think of pH as a number line from 0 to 14. It's like a temperature scale for acidity and basicity. Below 7 is acidic (like vinegar), 7 is neutral (like pure water), and above 7 is basic (like soap). 📏
Neutralization
When acids and bases meet, they cancel each other out, forming water and salt. It's like mixing hot and cold water to get warm water - they balance each other. This is why we use basic substances to treat acidic stomach problems. 🔄
範例
- When you squeeze lemon juice (an acid) into tea and it tastes sour, that's acid at work. If you add too much, you can balance it with a spoon of sugar (slightly basic) to make it taste better. 🍵
- When your stomach feels acidic (too much stomach acid), you take an antacid tablet (a base) to feel better. The base neutralizes the excess acid. 💊
- Using soap (a base) to wash greasy dishes works because bases can break down oils and fats (which are slightly acidic). This is why soap feels slippery! 🧼
費曼AI如何引導你學習
- 選擇任意概念: 從你想掌握的主題開始——瀏覽精選學科或自行輸入。
- 先學核心要點: 用清楚、結構化的解說快速建立知識框架,掌握關鍵與常見誤區。
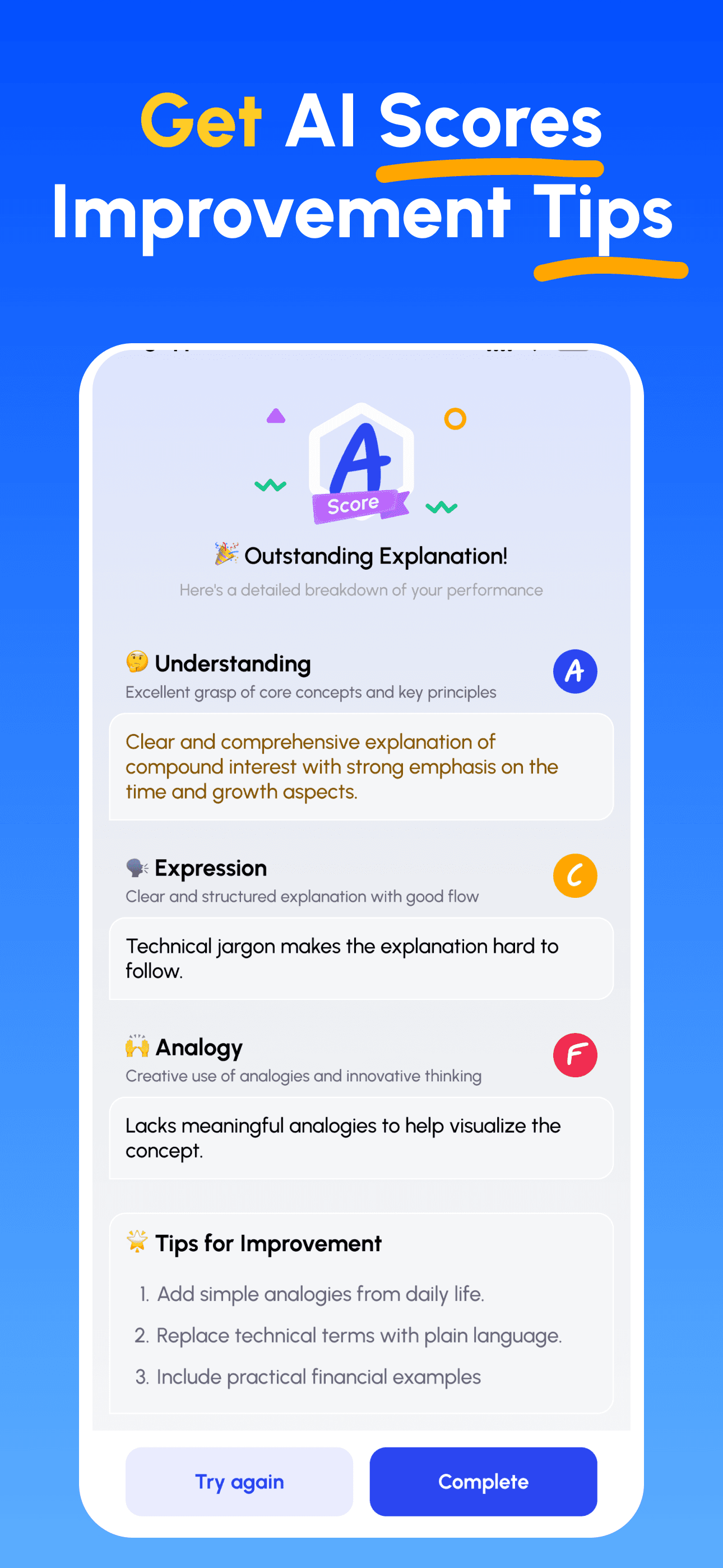
- 講解並獲得回饋: 以語音或文字錄製你的講解;立即取得在深度、清晰度、結構與示例上的分析。
- 檢視評分並精進: 依據針對性建議修正並再講解,直到能簡單講清楚為止。
立即下載費曼AI
今天就開始提升溝通能力的旅程!