Acid and Base
Acids are sour substances that give up protons, while bases are bitter substances that accept protons.
Brief Introduction
Acids and bases are two fundamental types of chemicals that we encounter daily, from the citrus fruits we eat to the soap we use. They're like opposite teams in chemistry: acids are substances that can give away hydrogen ions (like sharing a card), while bases are substances that can accept these ions (like collecting cards). Understanding acids and bases helps us make sense of many everyday processes, from cooking to cleaning. 🧪
Main Explanation
Properties and Behavior
Acids taste sour (like lemons), turn blue litmus paper red, and feel slippery. It's like they're the 'sharp' team. Bases taste bitter (like soap), turn red litmus paper blue, and feel smooth. They're like the 'smooth' team. 🍋
pH Scale
Think of pH as a number line from 0 to 14. It's like a temperature scale for acidity and basicity. Below 7 is acidic (like vinegar), 7 is neutral (like pure water), and above 7 is basic (like soap). 📏
Neutralization
When acids and bases meet, they cancel each other out, forming water and salt. It's like mixing hot and cold water to get warm water - they balance each other. This is why we use basic substances to treat acidic stomach problems. 🔄
Examples
- When you squeeze lemon juice (an acid) into tea and it tastes sour, that's acid at work. If you add too much, you can balance it with a spoon of sugar (slightly basic) to make it taste better. 🍵
- When your stomach feels acidic (too much stomach acid), you take an antacid tablet (a base) to feel better. The base neutralizes the excess acid. 💊
- Using soap (a base) to wash greasy dishes works because bases can break down oils and fats (which are slightly acidic). This is why soap feels slippery! 🧼
How Feynman AI Guides Your Learning
- Choose Any Concept: Start from a topic you want to master — browse curated subjects or enter your own.
- Learn Essentials: Skim clear, structured explanations, key terms and common pitfalls to form a solid mental model.
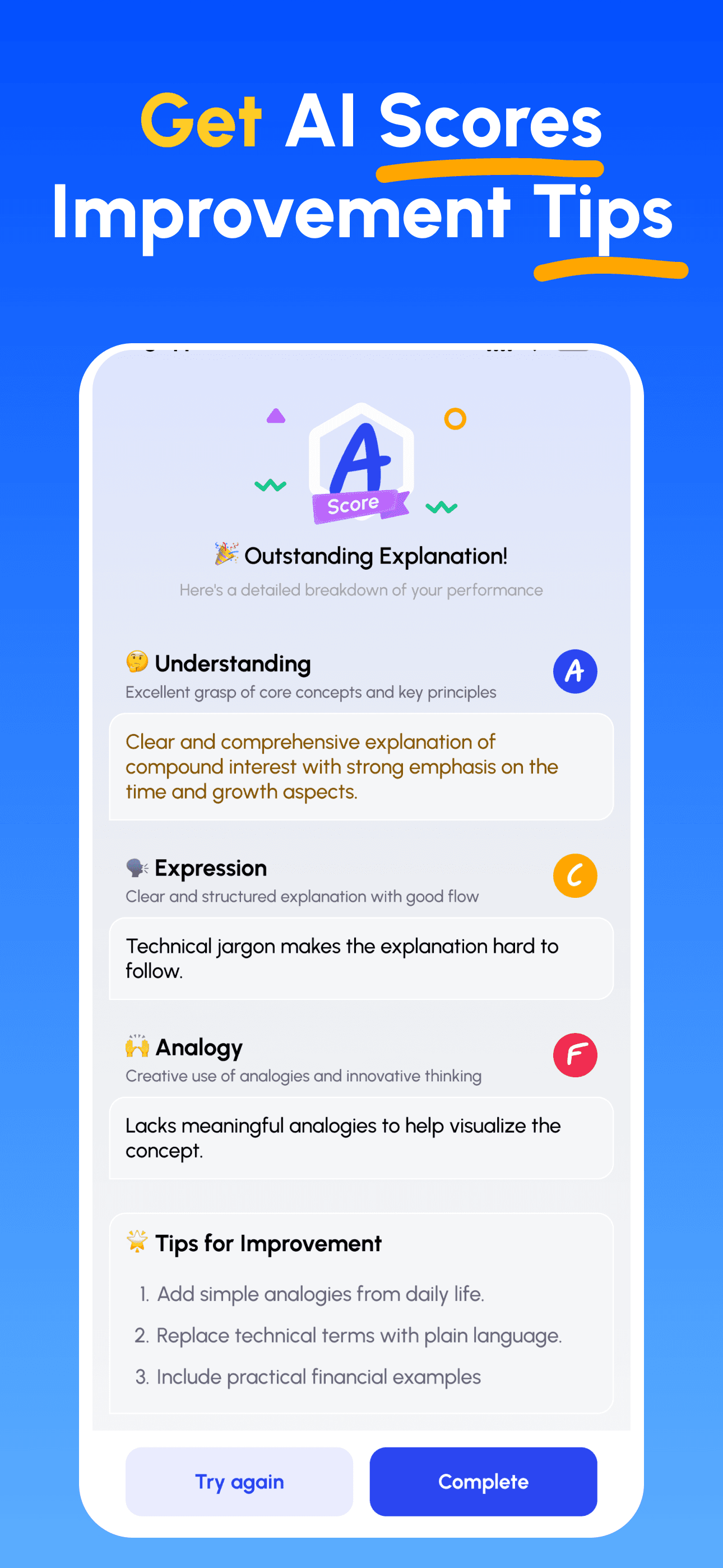
- Explain & Get Feedback: Record your explanation (voice or text). Get instant analysis on depth, clarity, structure and example quality.
- Review Scores & Improve: Follow targeted tips, refine your explanation and iterate until you can teach it simply.
Download Feynman AI Now
Start your learning journey today!